usp class vi materials
The USP outlines classes for plastic materials ie. In these critical applications reliable sealing is paramount to ensuring cleanliness and maintaining leak-free integrity against a wide range of gases and environmental exposures.

Usp Class Vi And Biocompatibility Of Products For Pharmaceutical Use Mtg
USP Class testing is one of the most common methods of testing to determine bio-compatibility of materials.

. So does ISO 10993. Our high-quality USP Class VI gaskets are specifically designed to meet the stringent requirements of the pharmaceutical biopharmaceutical and biotechnology industries. Most importantly use of Class VI certified materials substantially reduces the risk of causing harm or increased stress to a patient from reaction to a toxic material.
Compliance to USP Class VI is often requested by users in the biopharmaceutical and medical industries. Materials Meets USP Class VI requirements Animal Derivative Free resin ADF Heat deflection temp 66psi 210F Low moisture absorption Translucent for viewing liquid level Easy consistent fabrication Applications Biocontainer bag transport totes Soak tanks Animal feeding and bedding carts Sterilization trays Carboys Configurations Sheet. Pharmacopoeia Class VI judges the suitability of plastic material intended for use as containers or accessories for parenteral preparations.
Medical grade plastic materials that meet FDA and USP Class VI requirements are available from Professional Plastics. Purified Water Systems AmbientHot HeatChemicalOther sterilization methods Clean-in-Place CIP Distribution Systems. We have the biopharmaceutical gaskets you need for.
USP Grade testing is one of the most common testing methods for determining the biocompatibility of materials. Should you have any questions please contact Desmond Hunt PhD. Class VI testing is aimed to certify that there are no harmful reactions or long-term bodily effects caused by chemicals that leach out of plastic materials.
However Class VI also requires subacute toxicity and implantation effects which many ISO 10993 categories do not. This is then injected into a specimen that is alive in order to see how it responds. High-purity plastic materials are used in applications from Medical Wands to Sterilization Trays and Endoscopic Probes Devices.
The FDA requires testing of finished devices however the demonstration of biocompatibility of materials according to USP Class VI standards is provided as an aid to device manufacturers in their material selection process. Class VI tests aim to document the absence of harmful reactions or long-term bodily effects caused by chemicals leached from plastic materials. Moldable polyurethanes Resilon4300 and 4301 Molythane4615 Machinable polymer-filled 0618 PTFE.
USP Class VI demands an intracutaneous irritation test. These USP Standards are set for quality purity strength and consistency and published in the US Pharmacopeia and the National Formulary USP NF. The CVI Series meets the specific needs of pharmaceutical equipment manufacturers who seek the assurance of USP Class VI compound certification for their inflatable seals.
Professional Plastics General Product Range Brochure. In 1988 in vitro tests were explored and USP concluded that in vitro. Our high-quality USP Class VI gaskets and O-rings are specifically designed to meet the stringent requirements of the pharmaceutical biopharmaceutical and biotechnology industries.
The Revision Bulletin will be incorporated in USP 41NF 36. USP Class VI Testing is only one standard of biocompatibility however. The USP Class VI compounds must be made from ingredients with clear histories of biocompatibility that meet.
What is the PEEK stand for. Pharmacopoeia USP Class VI outlines requirements for system toxicity and intracutaneous toxicity for these cleaner compounds. There are six classes VI being the most rigorous.
Features Benefits 7 USP Class VI materials EPDM silicone fluorocarbon and perfluoroelastomer 24 materials which are compliant to FDA 21 CFR1772600 Specially formulated for long term sealing Compounds made without animal-derived ingredients BSETSE concerns Previous Next. A number of our plastic materials are ISO-10993 or USP Class VI capable. Suitability under USP Class VI is typically a base requirement for medical device manufacturers.
Its possible that a USP Class VI material can also comply ISO 10993. Yet some suppliers that use compliant ingredients may still not be able to guarantee a compliant end-product. The Plastic Packaging Systems and Their Materials of Construction Revision Bulletin will supersede the monograph becoming official in USP 40NF 35.
Our USP Class VI certified material offering includes. The CVI Series combines use of the right materials for biocompatibility with the unique. 1965 USP XVII introduced Biological TestsPlastics Containers section was added and made official in the Compendium.
What Are the USP VI Testing Methods. It generally ensures a high quality level and better acceptance with the FDA and USDA. USP Class VI Approved Plastic Materials USP US.
27 rows The US. The materials listed below are. Plastics were assigned Class I-VI based on the biological in vivo testing systemic injection intra-cutaneous and implantation tests.
Testing is carried out through producing an extract of the product using different extraction fluids which can include Polyethylene Glycol and vegetable oil. I - VI with USP Class VI being the strictest requiring that the material exhibit very low levels of toxicity proven through a series of tests. A new line of inflatable seals which meet US Pharmacopoeia USP Class VI certification is now available.
These products perform well even after autoclaving cycles. We have the O-rings you need for. Medical certifications as well as lot batch traceability are available.
That said the lack of risk assessment in USP Class VI can be a problem. There are six classes VI being the strictest. A selection of Figure 4 VisiJet Accura and DuraForm plastic materials have met the requirements of ISO 10993-5 -10 or USP Class VI testing.
Purified Water Systems AmbientHot HeatChemicalOther sterilization methods Clean-in-Place CIP Distribution Systems. USP class testing standards are set by the. USP Class VI materials meet the most stringent requirements and include silicones that pass a systemic toxicity test an intracutaneous test and an implantation test.
Usp31nf26s1 C1031 General Chapters 1031 The Biocompatibility Of Materials Used In Drug Containers Medical Devices And Implants
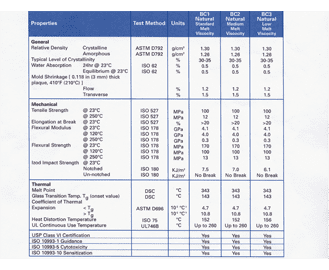
Bedeutung Von Usp Class Vi Standard Germany
Usp31nf26s1 C1031 General Chapters 1031 The Biocompatibility Of Materials Used In Drug Containers Medical Devices And Implants
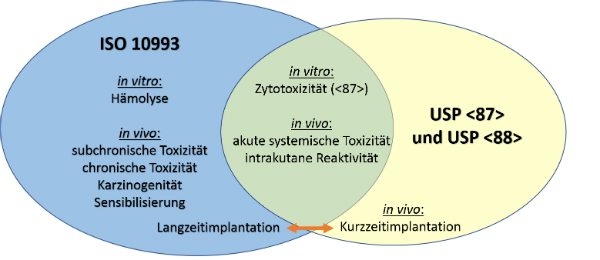
Biokompatibilitat Ein Massstab Fur Usp Class Vi Reichelt Chemietechnik Magazin

Bedeutung Von Usp Class Vi Standard Germany

Understanding Food Grade Vs Biocompatibility For Medical Device Materials Medical Product Outsourcing

Was Bedeuten Usp Class Vi Und Adi Frei Fur Ihren Bioprozess Brooks Instrument

What Is Usp Class Vi Testing Tbl Plastics
Usp Class Vi Zulassung Was Bedeutet Das Reichelt Chemietechnik Magazin

Usp Class Plastics Pacific Biolabs

Material Selection Medical Injection Molding Xcentric Mold

Usp Class Vi Zulassung Was Bedeutet Das Reichelt Chemietechnik Magazin

O Rings Fda And Usp Class Vi Darcoid Rubber Company Oakland California

Usp Class Vi Foster Corporation

Usp Class Vi Zulassung Was Bedeutet Das Reichelt Chemietechnik Magazin



